Fundamental Guide to Boiler Water Treatment: Essential Practices Every Power Plant Engineer Must Know
Introduction: Why Boiler Water Treatment Makes or Breaks Your Boiler
Pinkman’s phone buzzed at 3:47 AM on a cold Tuesday morning. As the newest engineer at the plant, he’d been dreading this moment—his first emergency call. The control room operator- Skinny Pete’s voice was tense: “We’ve got steam quality issues, and the turbine protection systems are acting up. Can you get here fast?”
Racing through empty streets, Pinkman’s mind raced through possibilities. When he arrived at the plant, the seasoned shift supervisor, Mr. White, was already pulling water samples. “Look at this,” he said, holding up a vial of what should have been crystal-clear condensate. Instead, it looked like weak tea. “This is what happens when boiler water treatment goes sideways, and it’s been building for weeks while nobody was watching the right signals.”
Now, before you think this is just another TV script: the characters are borrowed, but the story is straight from the realities of power generation. Here’s what nobody tells you when you start in power generation: water is both your best friend and your worst enemy. In its purest form, it’s the perfect working fluid—abundant, non-toxic, and incredibly efficient at absorbing and releasing energy. But introduce even tiny amounts of the wrong substances, and that same water becomes a destructive force that can eat through steel, form rock-hard deposits, and turn precision turbine blades into scrap metal.
Think about it this way—your boiler system processes thousands of gallons of water every hour, concentrating trace impurities through repeated evaporation cycles. It’s like making a pot of soup and reducing it down over and over again. What starts as barely detectable seasoning eventually becomes an inedible salt block. Except in your boiler, that “salt block” forms inside expensive tubes and components that were never designed to be replaced frequently.
By the time you finish reading this guide, you’ll understand why Mr. White could look at a single water sample and know exactly what had been going wrong for weeks. We’ll transform you from someone who might see water treatment as a “black box” operation into a confident engineer who understands every aspect of this critical process. You’ll learn not just what to do, but why each step matters, how different components work together, and most importantly, how to troubleshoot when things go wrong. More than anything, you’ll learn to see water treatment through the eyes of someone who understands that every drop processed today affects tomorrow’s operational reliability.
Understanding the Enemy: What Makes Untreated Water Dangerous
Before we dive into solutions, let’s understand exactly what we’re fighting against. Raw water, whether from rivers, lakes, or wells, contains numerous impurities that can wreak havoc on boiler systems. Think of these impurities as unwanted guests at a carefully orchestrated party—each one brings its own set of problems.
- Dissolved solids are perhaps the most insidious enemies. These invisible minerals, including calcium, magnesium, sodium, and silica, seem harmless in small concentrations but become concentrated as water evaporates in the boiler. Imagine adding a teaspoon of salt to a glass of water, then evaporating half the water—suddenly, that mild saltiness becomes overwhelming. The same principle applies in boilers, where dissolved solids concentrate and eventually precipitate out as hard, heat-resistant scales.
- Suspended particles are the visible troublemakers—dirt, silt, organic matter, and metal oxides that cloud the water. While easier to spot than dissolved solids, these particles create their own problems by settling in low-flow areas, plugging tubes, and providing nucleation sites for scale formation. They’re like seeds that encourage bigger problems to grow.

- Dissolved gases, particularly oxygen and carbon dioxide, accelerate corrosion processes throughout the system. Oxygen acts like a catalyst for metal oxidation, while carbon dioxide forms weak acids that attack metal surfaces. These gases are especially dangerous because they remain dissolved in water until temperature and pressure changes cause them to come out of solution, often at the worst possible locations.
- Organic matter and microbiological contaminants introduce another layer of complexity. Bacteria, algae, and organic compounds can form biofilms, create localized corrosion cells, and produce acidic byproducts that attack metal surfaces. They also interfere with chemical treatment programs and create operational headaches that are difficult to diagnose.
The domino effect of poor water quality starts small but quickly escalates. Scale formation begins as microscopic deposits that reduce heat transfer efficiency. As little as 1 millimeter of scale can reduce heat transfer by 5-10%, forcing operators to increase firing rates to maintain steam output. This higher heat flux accelerates scale growth, creating a vicious cycle that ends with tube failures, forced outages, and expensive repairs.
Corrosion follows a similar pattern, starting as microscopic pits that gradually grow into through-wall failures. The insidious nature of corrosion is that it often occurs in hidden locations—under deposits, in crevices, or in areas with stagnant flow—making it difficult to detect until significant damage has occurred.

Understanding these mechanisms isn’t just academic exercise. When you can visualize how impurities behave in your system, you’ll make better decisions about treatment strategies, monitoring priorities, and maintenance scheduling. You’ll also develop the intuition needed to troubleshoot problems quickly and effectively.
Boiler Water Quality Standards: The Non-Negotiable Benchmarks
Every power plant engineer needs to understand that water quality standards are carefully established limits based on decades of experience and scientific research. Think of them as the guardrails that keep your plant running safely and efficiently.
- pH levels represent one of the most critical parameters you’ll monitor. For most drum-type boilers, the ideal pH range of 8.5-9.5 represents a careful balance between corrosion protection and avoiding caustic attack. Below 8.5, acidic conditions promote general corrosion of steel components. Above 9.5, caustic conditions can cause embrittlement and gouging, particularly in high-heat-flux areas. This narrow window might seem restrictive, but it’s based on extensive metallurgical studies showing where steel surfaces remain most stable.
- Conductivity serves as your early warning system for dissolved solids contamination. Specific conductivity measurements tell you how many ions are dissolved in your water, while cation conductivity (after removing alkali metals) reveals the presence of harmful contaminants like chlorides and sulfates. Think of conductivity as a rapid screening test—when values start climbing, you know something has changed in your water chemistry, even before individual ion concentrations exceed limits.
- Silica content deserves special attention because it’s one of the most damaging contaminants for steam turbines. Silica volatilizes with steam and deposits on turbine blades, creating rough surfaces that reduce efficiency and can lead to vibration problems. The solubility of silica decreases with temperature, so what seems like an acceptable level in the boiler can become a serious problem in the superheater and turbine sections.
- Total dissolved solids (TDS) and cycles of concentration work together to help you manage boiler blowdown requirements. As water evaporates to form steam, dissolved solids concentrate in the remaining boiler water. The cycles of concentration tell you how much more concentrated your boiler water is compared to feedwater. Higher cycles mean better water utilization but require tighter control to prevent exceeding solubility limits.
- Dissolved oxygen targets vary significantly based on system design and operating conditions. For deaerating systems, oxygen levels below 7 parts per billion (ppb) are typical, while non-deaerating systems might accept higher levels with appropriate chemical scavenging. The key is understanding that even trace amounts of oxygen can cause significant corrosion over time.
- Pressure-specific requirements reflect the reality that higher-pressure systems are less forgiving of water quality deviations. Low-pressure boilers (below 15 bar) can tolerate higher dissolved solids concentrations because lower temperatures reduce the driving force for scale formation and corrosion. Medium-pressure systems (15-40 bar) require tighter specifications as temperatures and heat fluxes increase. High-pressure boilers (above 40 bar) demand ultra-pure water because even minor impurities can cause significant problems at these severe operating conditions.
- Supercritical units operate under such extreme conditions that water quality requirements approach those used in pharmaceutical manufacturing. At these pressures and temperatures, there’s no margin for error, and impurities that might be tolerable in conventional units become serious threats to equipment integrity.
Understanding these standards helps you prioritize your monitoring efforts and recognize when system changes require adjustments to water treatment programs. Remember, these are minimum requirements—many successful plants operate with water quality significantly better than required standards, viewing superior water treatment as insurance against unexpected problems.
>>> Checkout Water Quality Association for more standards to maintain in your boiler.
Pre-Treatment Systems: Your First Line of Defense
Pre-treatment systems are where the battle for water quality begins, and getting this foundation right makes everything downstream much easier to manage. Think of pre-treatment as the bouncer at an exclusive club—its job is to keep the troublemakers out before they can cause problems inside.
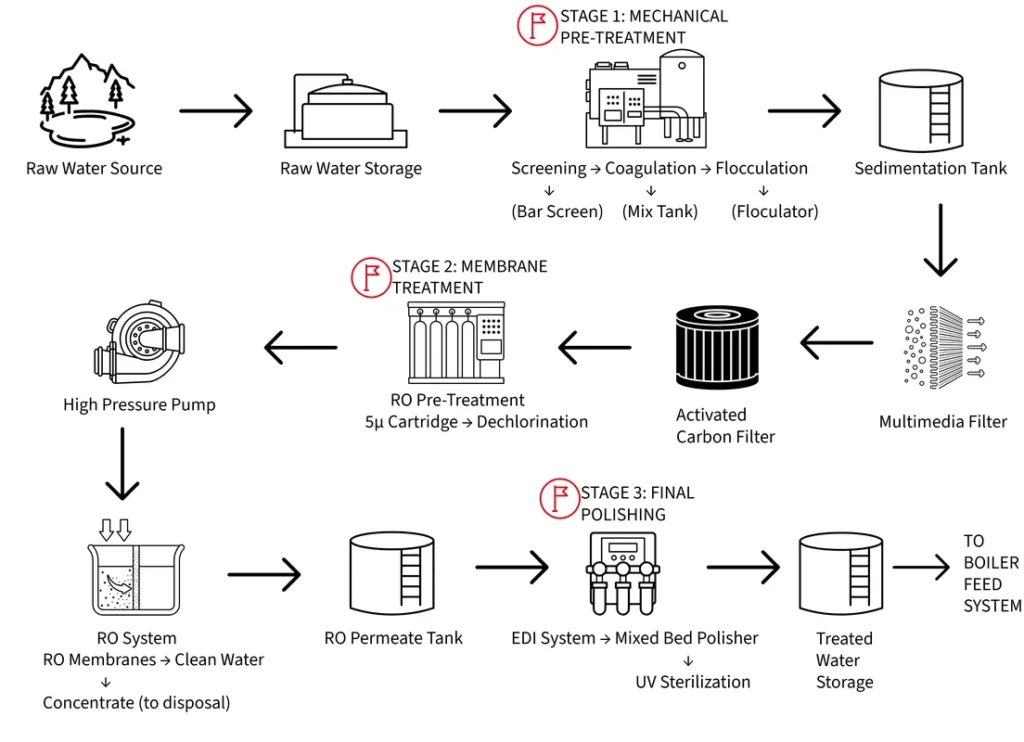
Understanding Your Water Source
Every water source has its own personality. Surface water (rivers, lakes) brings high suspended solids and organic matter but generally lower dissolved minerals. Groundwater typically has higher dissolved solids but fewer particles and organics. Understanding your source helps you design treatment systems that address specific challenges.
If your source has seasonal turbidity from agricultural runoff, you’ll need robust clarification with peak capacity. If groundwater has high dissolved solids, you’ll need aggressive demineralization.
Mechanical Treatment: The Physical Cleanup
Mechanical treatment processes remove suspended solids through physical and chemical means. Clarification systems use coagulation and flocculation to gather small particles into larger, settable masses. The chemistry here is fascinating—coagulants like aluminum sulfate or ferric chloride neutralize the electrical charges that keep particles suspended, allowing them to collide and stick together. Flocculants, usually long-chain polymers, provide the “glue” that holds these masses together as they grow into settleable flocs.
Sedimentation tanks provide time and space for gravity separation. The key is overflow rate—if water moves upward faster than flocs can settle, they escape into your clean water.
Filtration systems provide final polishing:
- Cartridge filters: Expensive but excellent for specific contaminant removal
- Sand filters: Work through straining and adsorption
- Multimedia filters: Use layered materials (coal/sand/garnet) for maximum dirt capacity
Advanced Technologies: The Game Changers
Advanced pre-treatment technologies have revolutionized water treatment in recent decades. Reverse osmosis (RO) systems represent perhaps the most significant advancement, capable of removing over 95% of dissolved solids along with virtually all suspended matter and organic compounds. Understanding RO requires grasping the concept of osmotic pressure—the natural tendency of water to move through a semipermeable membrane from areas of low dissolved solids concentration to areas of high concentration.
RO systems overcome this natural tendency by applying pressure greater than the osmotic pressure, forcing water molecules through the membrane while leaving contaminants behind. The quality of RO permeate is so high that it often requires remineralization to prevent corrosion in downstream systems.
Membrane selection depends on the specific contaminants you need to remove and the chemical compatibility with your cleaning protocols. Cellulose acetate membranes offer excellent chlorine tolerance but have pH and temperature limitations. Polyamide membranes provide superior rejection and wider pH tolerance but require dechlorination to prevent oxidative damage.
Recovery rates—the percentage of feed water that becomes product water—directly impact both water efficiency and concentrate disposal costs. Higher recovery rates mean less waste but require more careful attention to scaling potential as dissolved solids concentrate in the reject stream.
Ultrafiltration/Microfiltration bridge the gap between conventional filtration and RO, providing excellent removal of suspended solids and bacteria while being less sensitive to fouling.
Electrodeionization (EDI) provides final polishing using ion-selective membranes and electrical current, producing mixed-bed quality water without chemical regeneration.
Pre-Treatment Technology Comparison Chart
| Technology | Removal Efficiency | Operating Cost | Maintenance | Best Applications | Limitations |
|---|---|---|---|---|---|
| Sand Filtration | 90-95% suspended solids | Low | Moderate | Surface water clarification | No dissolved solids removal |
| Multimedia Filtration | 95-99% suspended solids | Low-Medium | Moderate | High turbidity water | Limited organics removal |
| Reverse Osmosis | >95% all contaminants | High | High | High TDS reduction | Chlorine sensitivity |
| Ultrafiltration | >99% bacteria/viruses | Medium | Medium | Microbiological control | No dissolved solids removal |
| Ion Exchange | >99% targeted ions | Medium | High | Selective ion removal | Regeneration chemicals |
| EDI (Electrodeionization) | >99% ionic impurities | Medium-High | Low | Final polishing | Requires pre-treatment |
System Integration Essentials
Modern pre-treatment requires:
- Redundancy: Multiple parallel trains for maintenance flexibility
- Automation: Online monitoring with automatic adjustments
- Flow management: Proper sizing for normal and peak conditions
- Control integration: Real-time optimization based on water quality
The goal is producing consistent, high-quality water that protects your expensive downstream equipment while minimizing chemical usage and waste generation.
Chemical Treatment Programs: The Science of Water Conditioning
Chemical treatment represents the sophisticated chemistry that transforms pre-treated water into the ultra-pure resource your boiler requires. This is where art meets science, and understanding the underlying chemistry helps you optimize treatment programs for your specific conditions.
Treatment Philosophy: Two Main Approaches
Precipitation Programs deliberately form insoluble compounds within the boiler, then remove them through blowdown before they deposit on heat transfer surfaces. Think of it as controlled scaling—you create the deposits where you want them, then wash them away.
Chelation Programs use organic molecules to “grab” metal ions and hold them in solution, preventing scale formation entirely. It’s like putting handcuffs on troublemaker ions so they can’t cause problems.
Core Treatment Programs
Phosphate Treatment (Most Common for Drum Boilers)
Phosphate treatment programs represent the most widely used precipitation approach for drum-type boilers. The chemistry involves converting calcium and magnesium ions into insoluble phosphate compounds that can be removed through blowdown. The key is maintaining proper phosphate residuals throughout the boiler water volume while avoiding areas of high phosphate concentration that could lead to acid phosphate corrosion.
Coordinated phosphate programs maintain specific ratios between phosphate and caustic alkalinity, ensuring that pH remains in the acceptable range even in areas of high heat flux where local concentration occurs. Congruent phosphate programs use combinations of disodium and trisodium phosphate to maintain pH stability through the buffer action of the phosphate system itself.
Polymer Treatment (Modern Alternative)
Polymer treatment programs offer modern alternatives that often provide superior performance with lower chemical usage. These synthetic organic molecules work by crystal modification and dispersal mechanisms, interfering with scale crystal growth and keeping particles suspended for removal through blowdown. Polymers are particularly effective for silica and iron control, contaminants that don’t respond well to traditional phosphate treatment.
All-Volatile Treatment (AVT) (High-Pressure Systems)
All-volatile treatment (AVT) programs are designed specifically for high-pressure systems where any solid residue is unacceptable. These programs use only volatile chemicals—typically ammonia and hydrazine—that decompose or evaporate without leaving solid residues. While providing excellent protection for high-pressure systems, AVT programs require more sophisticated monitoring and control.
Water Quality vs. Treatment Program Matrix
| Water Quality Challenge | Precipitation Program | Chelation Program | All-Volatile Treatment | Polymer Program | Best for |
|---|---|---|---|---|---|
| High calcium/magnesium | ✅ Phosphate | ✅ EDTA/NTA | ❌ Not suitable | ✅ Acrylic polymers | Drum boilers |
| Silica control | ⚠️ Limited effectiveness | ✅ Specialized chelants | ❌ Not effective | ✅ Polyacrylates | All pressures |
| Iron/copper control | ✅ With dispersants | ✅ Metal chelation | ✅ Volatile reduction | ✅ Crystal modification | Varies by source |
| High-pressure systems | ❌ Solid residues | ❌ Decomposition issues | ✅ No solids | ⚠️ Decomposition products | >40 bar systems |
| Environmental concerns | ⚠️ Blowdown disposal | ⚠️ Chelant persistence | ✅ Volatile chemicals | ✅ Biodegradable options | Eco-sensitive locations |
Essential Chemical Categories
Oxygen Scavengers (Corrosion Protection)
Oxygen scavengers protect the entire steam-water cycle from corrosion by removing dissolved oxygen that remains after mechanical deaeration.
- Sodium sulfite represents the traditional choice, reacting rapidly with oxygen to form harmless sulfate ions. The reaction requires elevated temperatures to proceed quickly, making sulfite most effective in the boiler environment.
- Hydrazine offers more complete oxygen removal and provides additional benefits by reducing iron and copper oxides to metallic forms. However, hydrazine’s toxicity and potential carcinogenicity have led many plants to seek alternatives. Modern organic scavengers like diethylhydroxylamine (DEHA) provide excellent oxygen removal with much lower toxicity concerns.
- Catalyzed scavenger systems use small amounts of metal catalysts to accelerate oxygen removal reactions, allowing effective scavenging at lower temperatures and reducing chemical consumption. These systems are particularly valuable for mixed pressure plants where some oxygen removal must occur in lower-temperature sections of the cycle.
pH Control Agents
pH control agents maintain water chemistry within acceptable ranges throughout the steam-water cycle.
- Caustic soda (sodium hydroxide) provides rapid pH adjustment in boiler water but must be carefully controlled to prevent caustic attack on metal surfaces. The key is maintaining sufficient alkalinity for corrosion protection while avoiding excessive concentrations that could cause embrittlement.
- Ammonia serves dual roles as a pH control agent and oxygen scavenger in some systems. Its volatility allows pH control in both boiler water and steam condensate, but ammonia can also contribute to stress corrosion cracking in copper-bearing alloys and must be carefully balanced.
- Morpholine and cyclohexylamine are volatile organic amines used primarily for condensate system pH control. These chemicals distribute with steam and neutralize carbonic acid formed when carbon dioxide dissolves in condensate. Their volatility ratios can be adjusted to provide pH control throughout the condensate system.
Scale and Corrosion Inhibitors
Scale and corrosion inhibitors provide additional protection through specialized chemical mechanisms.
- Phosphonates work by crystal modification, interfering with scale crystal growth and promoting the formation of non-adherent deposits that can be removed through normal blowdown. These chemicals are particularly effective against calcium carbonate and calcium phosphate scales.
- Chelating agents like EDTA and NTA form stable complexes with metal ions, preventing scale formation entirely. While effective, these chemicals can be expensive and may interfere with other treatment chemicals, requiring careful program design and monitoring.
- Filming amines create protective films on metal surfaces, providing an additional barrier against corrosion. These chemicals are particularly valuable in condensate systems where traditional alkaline conditions cannot be maintained.
Chemical Treatment Selection Matrix
| System Pressure | Primary Concern | Recommended Program | Chemical Components | Monitoring Priority |
|---|---|---|---|---|
| <15 bar | Scale prevention | Phosphate precipitation | Na₃PO₄ + NaOH + Na₂SO₃ | pH, phosphate residual |
| 15-40 bar | Scale + corrosion | Coordinated phosphate | Na₃PO₄/Na₂HPO₄ + NaOH + DEHA | pH/phosphate ratio |
| 40-100 bar | Purity critical | Polymer + AVT | Polyacrylate + NH₃ + N₂H₄ | Cation conductivity |
| >100 bar | Zero solids | All-volatile treatment | NH₃ + N₂H₄ only | Cation conductivity, pH |
| Supercritical | Ultra-purity | Modified AVT | Ethanolamine + O₂ control | All parameters critical |
Chemical Feed System Essentials
Design Requirements:
- Injection points with adequate mixing before reactions occur
- Pump sizing for normal and upset conditions
- Redundancy to prevent treatment failures
- Variable-speed drives with automated control
Storage Considerations:
- Adequate inventory for normal operations
- Chemical compatibility and safety requirements
- Temperature control and emergency containment
- Integration with plant control systems for real-time adjustments
Monitoring and Testing: Your Early Warning System
Effective monitoring and testing programs serve as the eyes and ears of your water treatment system, providing the information needed to maintain optimal chemistry and detect problems before they become serious. Think of monitoring as preventive medicine for your boiler system—regular check-ups catch problems early when they’re easy to fix.
Boiler Water Treatment System Performance Tracking Table
| System Component | Key Performance Indicator | Normal Range | Warning Level | Action Required | Check Frequency |
|---|---|---|---|---|---|
| RO System | Permeate conductivity | <2 µS/cm | >5 µS/cm | Clean membranes | Daily |
| RO System | Recovery rate | 75-85% | <70% or >90% | Adjust operating pressure | Daily |
| Ion Exchange | Effluent conductivity | <1 µS/cm | >2 µS/cm | Regenerate resin | Continuous |
| Deaerator | O₂ in effluent | <7 ppb | >10 ppb | Check steam flow/packing | Shift |
| Chemical Feed | Tank levels | >25% | <25% | Reorder chemicals | Daily |
| Chemical Feed | Pump operation | Normal flow | Flow deviation >10% | Check pump/lines | Shift |
Monitoring Frequency and Action Levels Table
| Parameter | Normal Monitoring | Alert Level | Alarm Level | Emergency Action | Field Test Available |
|---|---|---|---|---|---|
| pH | Every 4 hours | ±0.2 from target | ±0.5 from target | Immediate chemical adjustment | ✅ Yes |
| Conductivi-ty | Continuous | 10% above normal | 25% above normal | Increase blowdown, investigate source | ✅ Yes |
| Dissolved O₂ | Every 8 hours | >5 ppb | >10 ppb | Check scavenger feed, increase dose | ⚠️ Special equipment |
| Silica | Daily | 50% of limit | 80% of limit | Reduce load, increase blowdown | ❌ Lab only |
| Iron | Weekly | 50% of limit | 80% of limit | Investigate corrosion source | ❌ Lab only |
| Chlorides | Every 4 hours | >0.1 ppm | >0.5 ppm | Find leak source, increase blowdown | ✅ Yes |
| Phosphate | Every 8 hours | ±20% of target | ±50% of target | Adjust chemical feed rate | ✅ Yes |
Troubleshooting Common Water Treatment Problems
Even the best-designed water treatment systems occasionally experience problems. Your ability to diagnose and correct these issues quickly can mean the difference between minor adjustments and major equipment damage. Effective troubleshooting requires systematic thinking and understanding of component relationships.
Scale Formation Scenarios
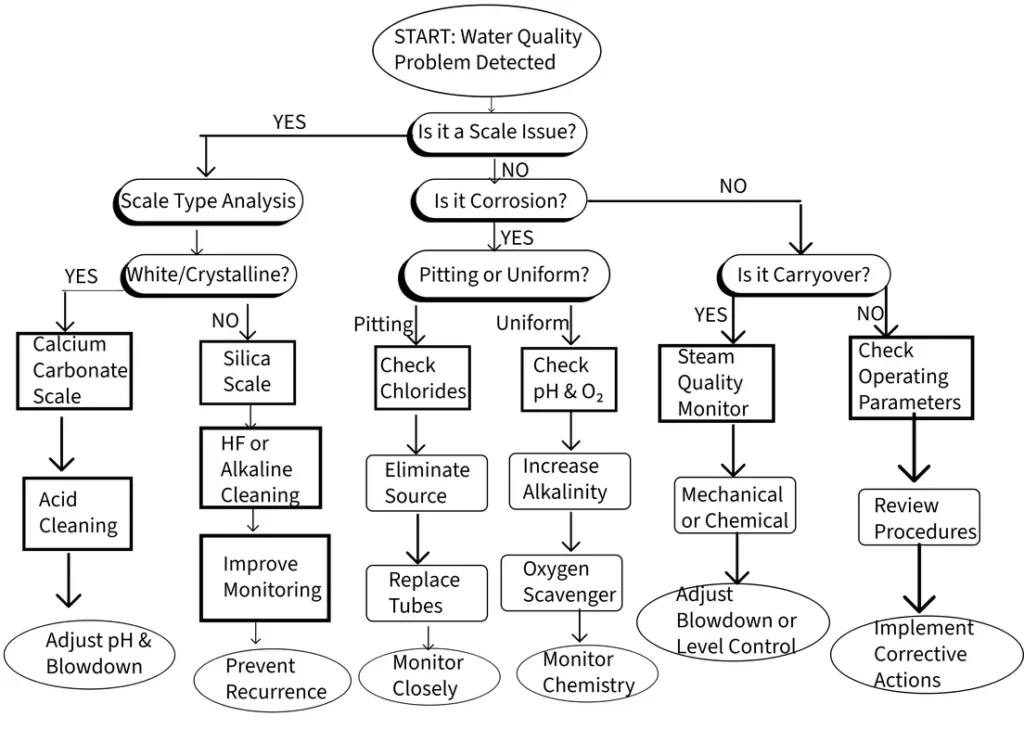
Scale formation represents one of the most common and potentially damaging problems you’ll encounter:
Calcium Carbonate Scale:
- Appearance: White, crystalline deposits
- Formation locations: High temperature areas, pH increase zones
- Characteristics: Soft when first formed, hardens with time/temperature
- Common causes:
- High calcium levels in feedwater
- pH increases due to CO₂ loss
- Inadequate chemical treatment
- Poor blowdown control
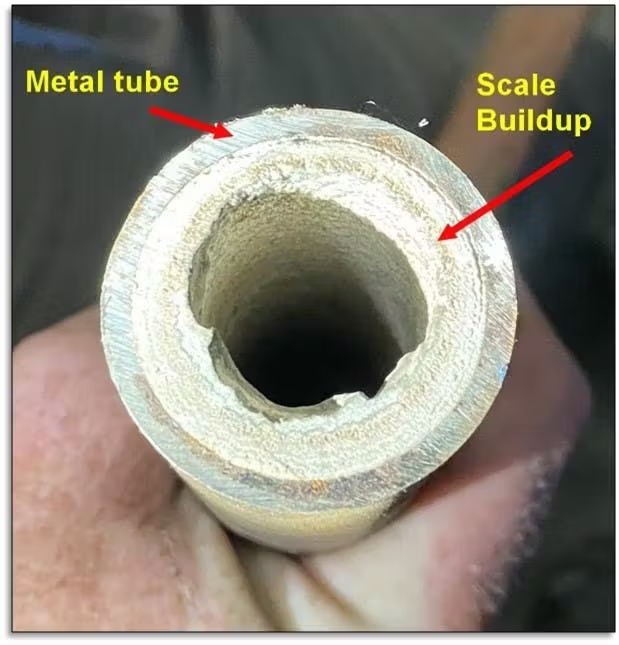
Silica Scale:
- Appearance: Glass-like, extremely hard deposits
- Formation locations: Superheater tubes, high-temperature areas
- Characteristics: Forms rapidly during upsets, nearly impossible to remove
- Common causes:
- Exceeded silica solubility limits
- Carryover from boiler water
- Inadequate pre-treatment
- Volatile silica formation

Phosphate Scale:
- Appearance: White to gray, hard crystalline deposits
- Formation locations: High heat-flux areas, nucleate boiling zones
- Characteristics: Often forms in areas where phosphate concentrates
- Common causes:
- Excessive phosphate feed rates
- Poor pH control
- Inadequate circulation
- Improper chemical program coordination
Cleaning Procedures
Calcium Carbonate Scale Removal: Calcium carbonate scales typically respond well to mild acid cleaning using inhibited hydrochloric or sulfamic acid. The key is using sufficient contact time at appropriate temperatures while preventing acid attack on base metals.
Silica Scale Removal: Silica scales require more aggressive treatment, often involving hydrofluoric acid or alkaline solutions at elevated temperatures. These cleaning procedures require special safety precautions and may have limitations based on metallurgy of system components.
Phosphate Scale Removal: Phosphate scales often respond to alkaline cleaning solutions that can dissolve phosphate compounds without attacking base metals. However, these cleaning procedures may require multiple cycles to achieve complete removal.
Corrosion Issues
Different corrosion mechanisms require specific diagnostic and treatment approaches:
Pitting Corrosion: Pitting corrosion typically results from localized breakdown of protective oxide films, often associated with chloride contamination or oxygen concentration cells. These attacks create deep, narrow pits that can quickly penetrate tube walls despite relatively low general corrosion rates.
Common causes:
- Chloride contamination
- Oxygen concentration cells
- Under-deposit corrosion
- Inadequate chemical protection
Prevention strategies:
- Eliminate chloride sources
- Maintain proper pH levels
- Ensure adequate oxygen scavenger residuals
- Regular deposit removal

Uniform Corrosion: Uniform corrosion results from general breakdown of protective films, typically due to low pH conditions or inadequate corrosion inhibitor concentrations. While this type of corrosion may appear less threatening because it’s distributed over large areas, it can still cause significant metal loss over time.
Common causes:
- Low pH conditions
- Inadequate corrosion inhibitor concentrations
- Oxygen contamination
- Acidic contaminants
Prevention strategies:
- Maintain proper alkalinity
- Adequate chemical treatment programs
- Effective oxygen removal
- Regular water chemistry monitoring
Caustic Gouging: Caustic gouging represents a specialized form of attack that occurs in high-heat-flux areas where caustic salts concentrate and attack protective oxide films. This type of attack is often associated with improper chemical treatment programs or inadequate circulation in steam-generating circuits.
Common causes:
- Excessive caustic concentrations
- Poor circulation in steam-generating circuits
- Inadequate blowdown
- Improper chemical treatment coordination
Prevention strategies:
- Control caustic/phosphate ratios
- Ensure adequate circulation
- Proper blowdown rates
- Coordinated chemical programs

Flow-Accelerated Corrosion (FAC): Flow-accelerated corrosion (FAC) occurs in single-phase water systems where protective oxide films are continuously removed by flowing water. This mechanism is particularly active in condensate and feedwater systems operating at specific temperature ranges where oxide film stability is marginal.
Common locations:
- Condensate systems
- Feedwater heaters
- Areas with high velocity flow
Prevention strategies:
- Maintain proper pH in affected systems
- Use appropriate metallurgies
- Control oxygen levels
- Regular thickness monitoring

>>> Checkout NACE International to learn more about corrosion prevention standards.
Carryover problems
Carryover problems occur when boiler water contaminants are transported with steam to downstream equipment.
Mechanical carryover results from inadequate steam-water separation in the steam drum, often caused by high water levels, excessive steam velocities, or inadequate internals design.
Detection methods:
- Steam purity monitoring
- Sodium analyzers in steam
- Visual observation of steam quality
Corrective actions:
- Adjust water level control
- Reduce steam generation rate
- Improve separation equipment
- Modify operating procedures
Chemical carryover occurs when dissolved solids volatilize with steam or are transported as microscopic droplets. This type of carryover is particularly dangerous for steam turbines because even small amounts of dissolved solids can cause deposits on turbine blades.
Detection methods:
- Cation conductivity in steam samples
- Specific ion analysis (sodium, chloride)
- Continuous steam purity monitoring
Corrective actions:
- Increase blowdown rates
- Reduce dissolved solids concentrations
- Eliminate foaming conditions
- Modify chemical treatment programs
Boiler Water Treatment Troubleshooting Flowchart
System Upsets and Emergency Responses
Predetermined procedures minimize equipment damage and restore normal operations:
Condenser Tube Leaks:
- Immediate actions:
- Reduce plant load to minimum safe level
- Increase blowdown rates to maximum safe limits
- Enhance monitoring frequency
- Isolate affected condenser sections if possible
- Monitoring priorities:
- Chloride levels in condensate and feedwater
- Conductivity trends
- pH stability
- Corrosion product levels
Chemical Feed System Failures:
- Assessment requirements:
- Determine affected chemical systems
- Calculate depletion rates for existing residuals
- Estimate safe operating time without feed
- Backup procedures:
- Manual chemical addition procedures
- Alternative feed point utilization
- Emergency chemical supply activation
- Load reduction if necessary
Raw Water Quality Excursions:
- Immediate evaluation:
- Assess pre-treatment system capacity
- Determine contamination severity
- Evaluate downstream impact potential
- Response actions:
- Increase pre-treatment chemical feed rates
- Reduce water production rates if necessary
- Implement enhanced monitoring
- Consider alternative water sources
Startup and Shutdown Procedures:
Startup and shutdown procedures present unique challenges because normal water chemistry control may not be achievable during these transient conditions. Startup procedures must account for the time required to establish proper chemical residuals and achieve normal operating temperatures and pressures.
Shutdown procedures should ensure adequate corrosion protection during idle periods while avoiding chemical programs that might interfere with restart operations. Understanding the different requirements for short-term versus long-term shutdowns helps optimize protection strategies.
- Startup considerations:
- Time required to establish chemical residuals
- Temperature and pressure ramp rates
- Monitoring frequency adjustments
- Equipment protection priorities
- Shutdown considerations:
- Corrosion protection during idle periods
- Chemical program modifications
- Duration-dependent procedures (short vs. long-term)
- Restart preparation requirements
Conclusion: Mastering the Art and Science of Boiler Water Treatment
As we conclude this comprehensive journey through boiler water treatment, it’s important to recognize that you’ve gained much more than technical knowledge—you’ve developed a systematic approach to understanding and managing one of the most critical aspects of power plant operations. The principles, practices, and troubleshooting methodologies covered in this guide will serve as your foundation for making sound engineering decisions throughout your career.
The critical success factors we’ve explored work together as an integrated system. Excellent pre-treatment protects downstream systems and reduces chemical costs. Proper chemical treatment programs prevent scale formation and corrosion while maintaining optimal heat transfer. Comprehensive monitoring and testing programs provide the information needed to maintain optimal chemistry and detect problems early. Effective troubleshooting skills help you diagnose and correct problems quickly, minimizing equipment damage and operational disruptions.
Take pride in choosing a field that combines scientific principles with practical problem-solving, where your daily work contributes to reliable electricity generation that powers modern life. The expertise you’re developing will serve you well, whether you remain focused on water treatment or expand into broader power plant operations and management roles.
The journey continues, and your foundation is strong. Build upon it with confidence, curiosity, and commitment to excellence.
~Rotormind




